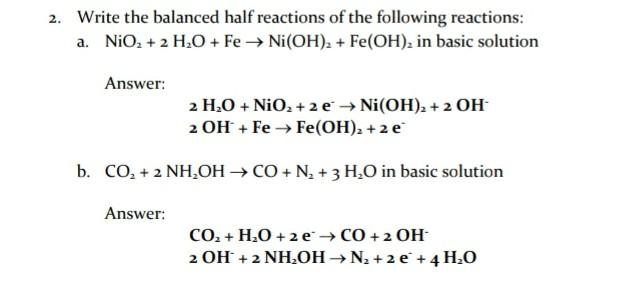Realizing Two-Electron Transfer in Ni(OH)2 Nanosheets for Energy Storage | Journal of the American Chemical Society

XRD patterns of (a) In(OH)3, (b) Ni(OH)2, (c) Ni(OH)2/In(OH)3, and (d)... | Download Scientific Diagram
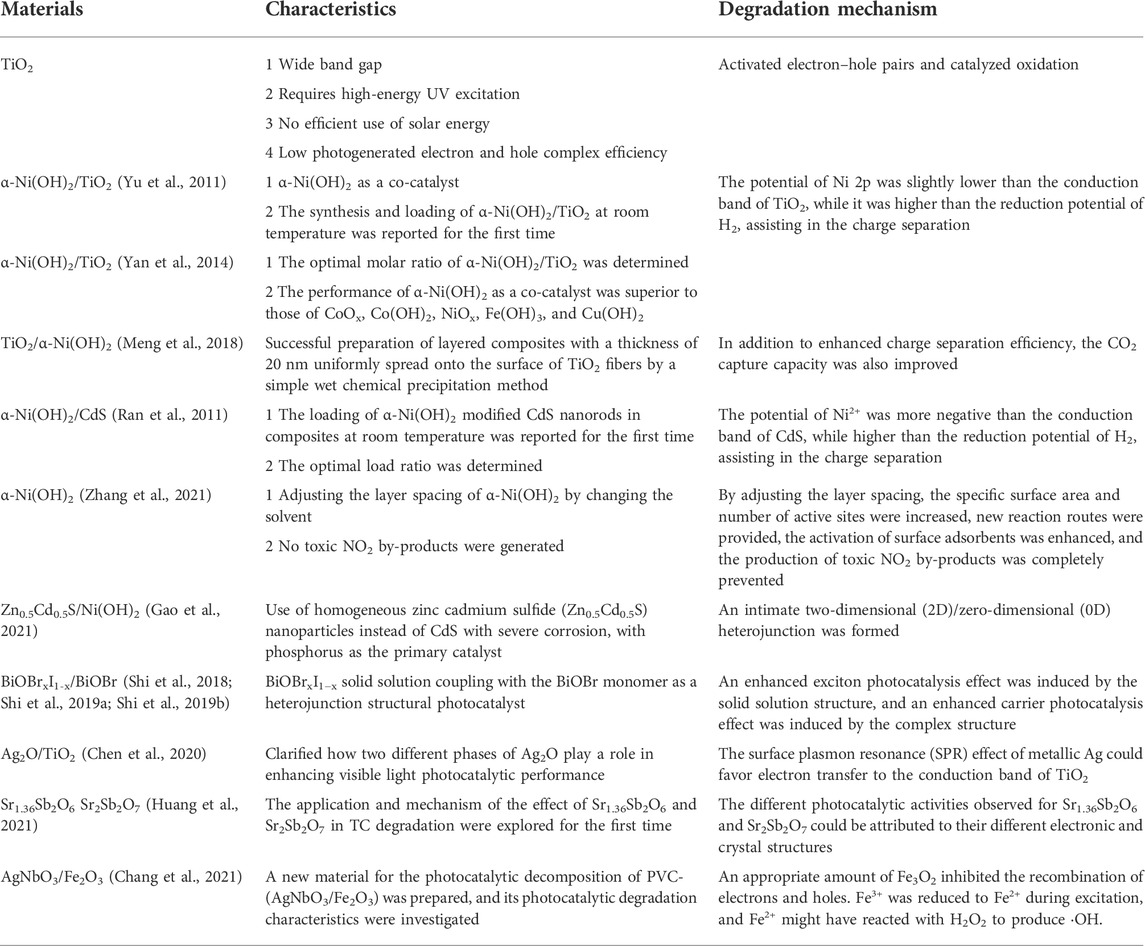
Frontiers | Tunable microscopic aggregation morphology of α-Ni(OH)2 for enhanced photocatalytic degradation of fracturing flowback fluid with ozone synergy

Facile preparation of three-dimensional Ni(OH)2/Ni foam anode with low cost and its application in a direct urea fuel cell - New Journal of Chemistry (RSC Publishing)
![The Role of the Redox Non‐Innocent Hydroxyl Ligand in the Activation of O2 Performed by [Ni(H)(OH)]+ - Kim - 2023 - Chemistry – A European Journal - Wiley Online Library The Role of the Redox Non‐Innocent Hydroxyl Ligand in the Activation of O2 Performed by [Ni(H)(OH)]+ - Kim - 2023 - Chemistry – A European Journal - Wiley Online Library](https://chemistry-europe.onlinelibrary.wiley.com/cms/asset/5a5d2b1b-25f4-4e6d-a83c-c65beeb759da/chem202203128-fig-0003-m.jpg)
The Role of the Redox Non‐Innocent Hydroxyl Ligand in the Activation of O2 Performed by [Ni(H)(OH)]+ - Kim - 2023 - Chemistry – A European Journal - Wiley Online Library

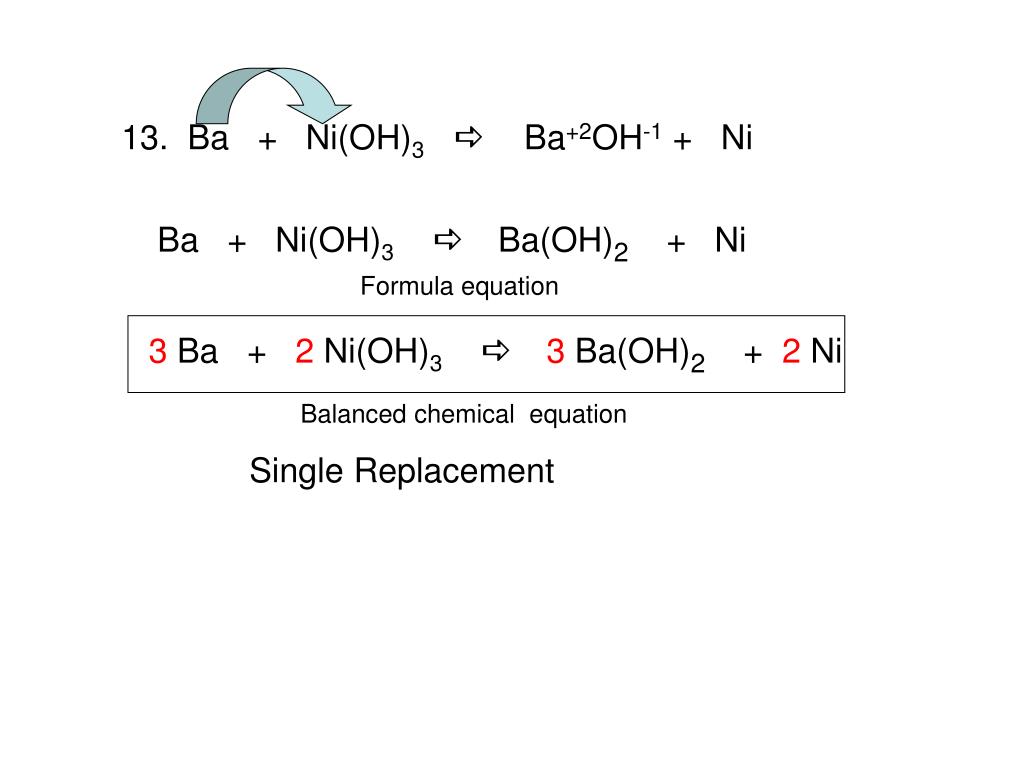
K of a salt Ni(OH), is 2 x10-15 then molar solubility of Ni(OH), in 0.01M NaOH is :- (1) 2 x 10-15 M (2) 21/3 x 10-5 M (3) 2 x 10-11 M (4) 107 M
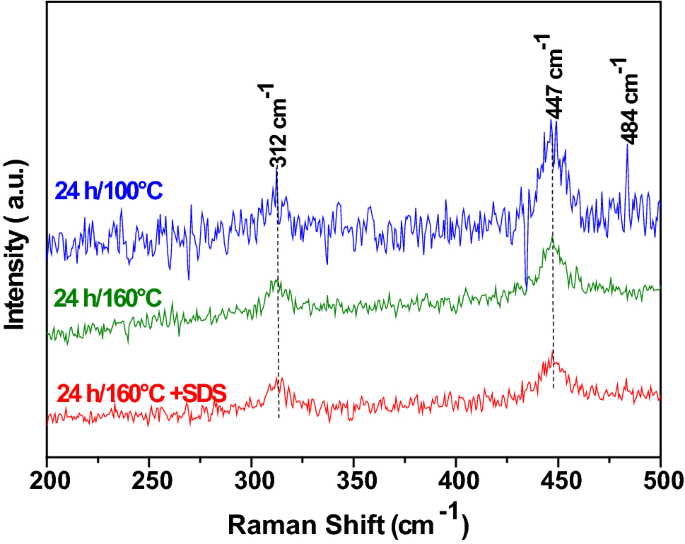
Electrochemical measurements of synthesized nanostructured β-Ni(OH)2 using hydrothermal process and activated carbon based nanoelectroactive materials | SN Applied Sciences
![1. Given the following equilibrium constants, calculate the solubility (moles/L) of Ni(OH)2(s) in a solution that has a fixed [OH-] of 3.2x10-7M Ni (OH)2(s) Ksp. - ppt download 1. Given the following equilibrium constants, calculate the solubility (moles/L) of Ni(OH)2(s) in a solution that has a fixed [OH-] of 3.2x10-7M Ni (OH)2(s) Ksp. - ppt download](https://slideplayer.com/15074812/91/images/slide_1.jpg)
1. Given the following equilibrium constants, calculate the solubility (moles/L) of Ni(OH)2(s) in a solution that has a fixed [OH-] of 3.2x10-7M Ni (OH)2(s) Ksp. - ppt download
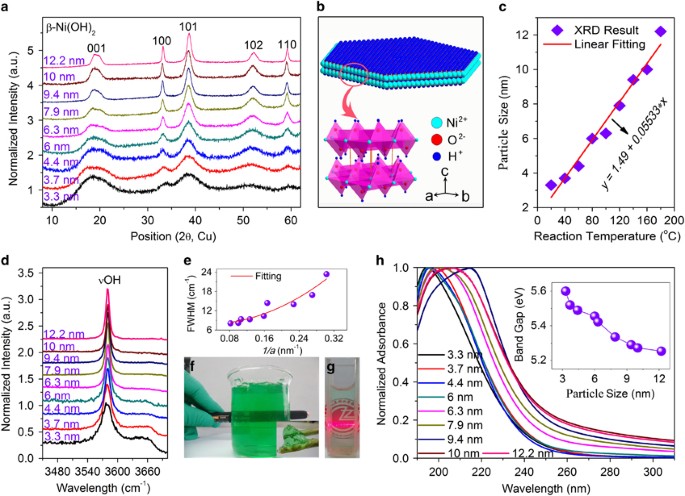
Ultra-small, size-controlled Ni(OH)2 nanoparticles: elucidating the relationship between particle size and electrochemical performance for advanced energy storage devices | NPG Asia Materials

Fabrication of Three-Dimensional Porous NiO/Amorphous Ni(OH)2 Composites for Supercapacitors | Energy & Fuels
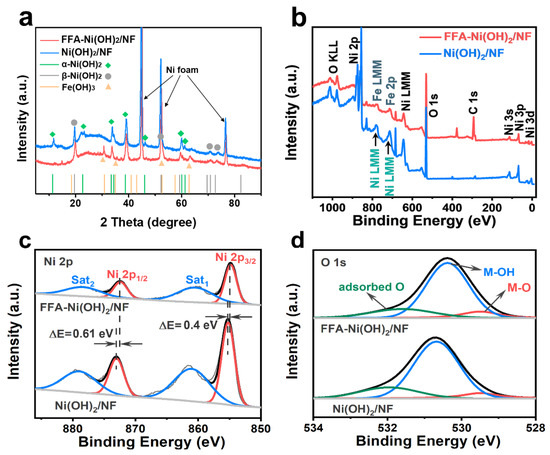
Crystals | Free Full-Text | Ferrocene Formic Acid Surface Modified Ni(OH)2 for Highly Efficient Alkaline Oxygen Evolution

Figure 3 from Electrochemical Performance of β-Nis@Ni(OH)2 Nanocomposite for Water Splitting Applications | Semantic Scholar

Catalysts | Free Full-Text | Catalysts Based on Ni(Mg)Al-Layered Hydroxides Prepared by Mechanical Activation for Furfural Hydrogenation
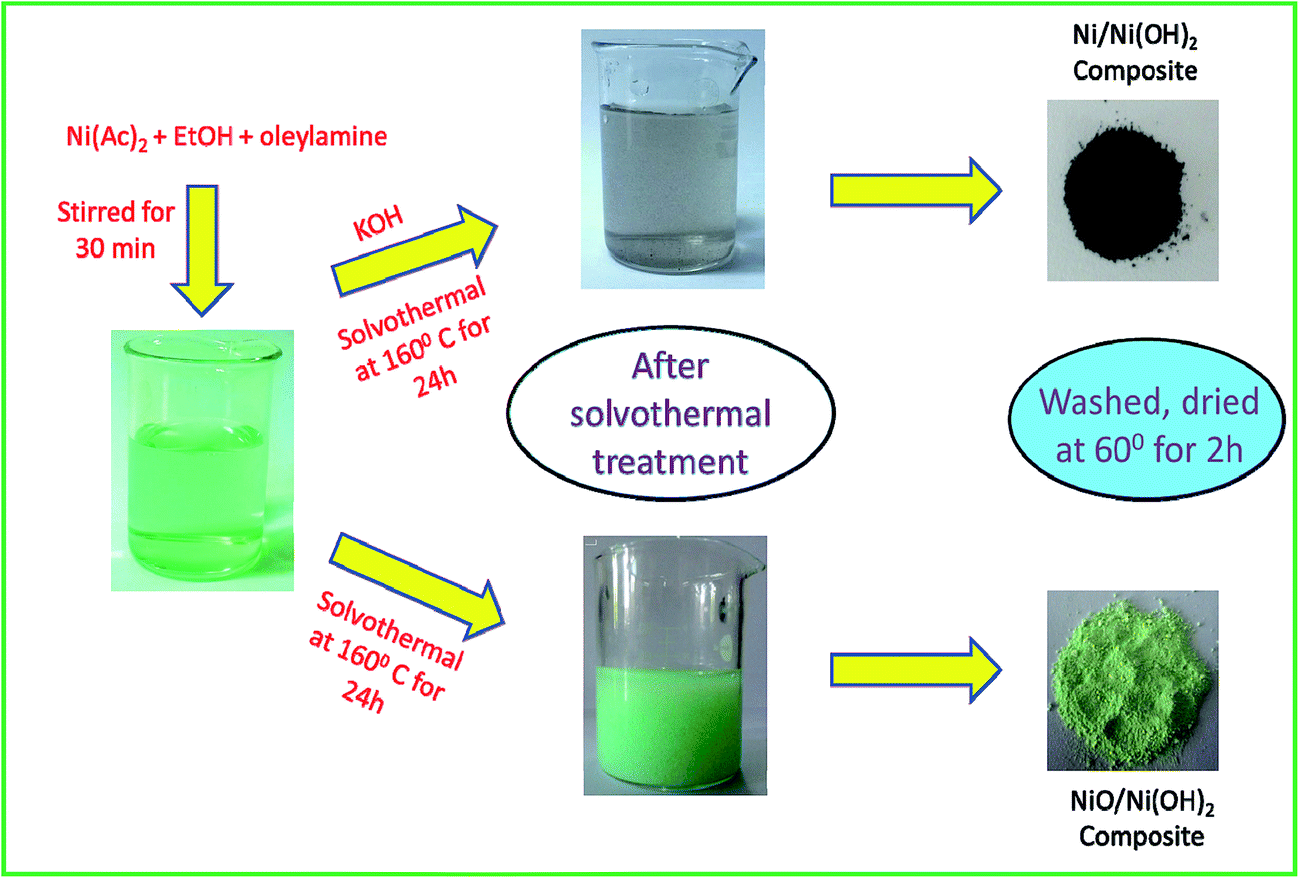
One step synthesis of Ni/Ni(OH) 2 nano sheets (NSs) and their application in asymmetric supercapacitors - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26584G

Nickel hydroxides and related materials: a review of their structures, synthesis and properties | Proceedings of the Royal Society A: Mathematical, Physical and Engineering Sciences