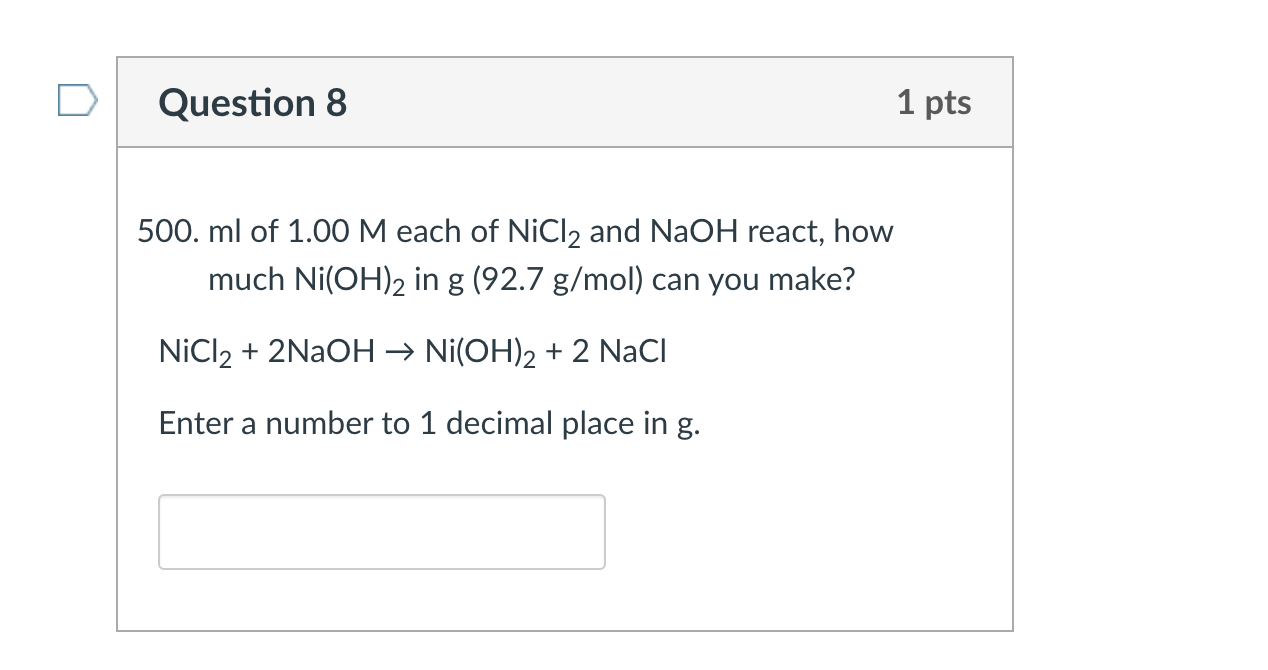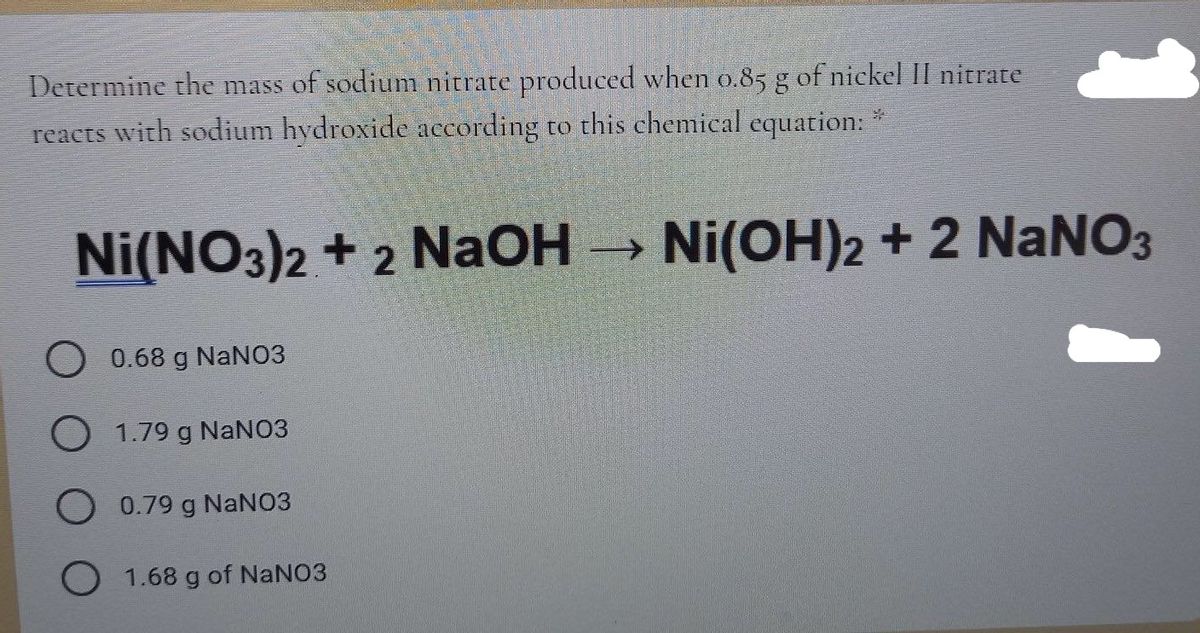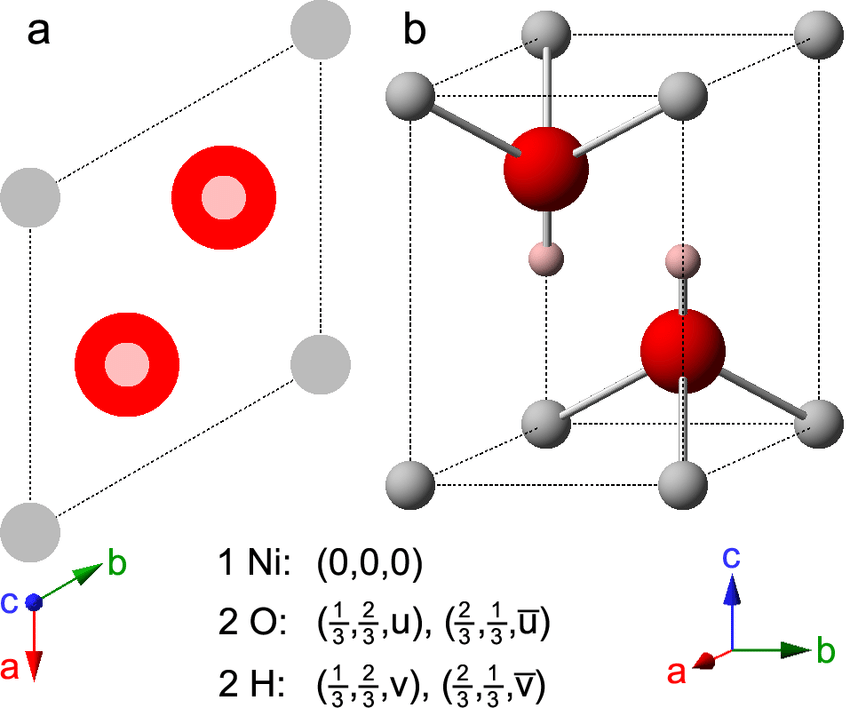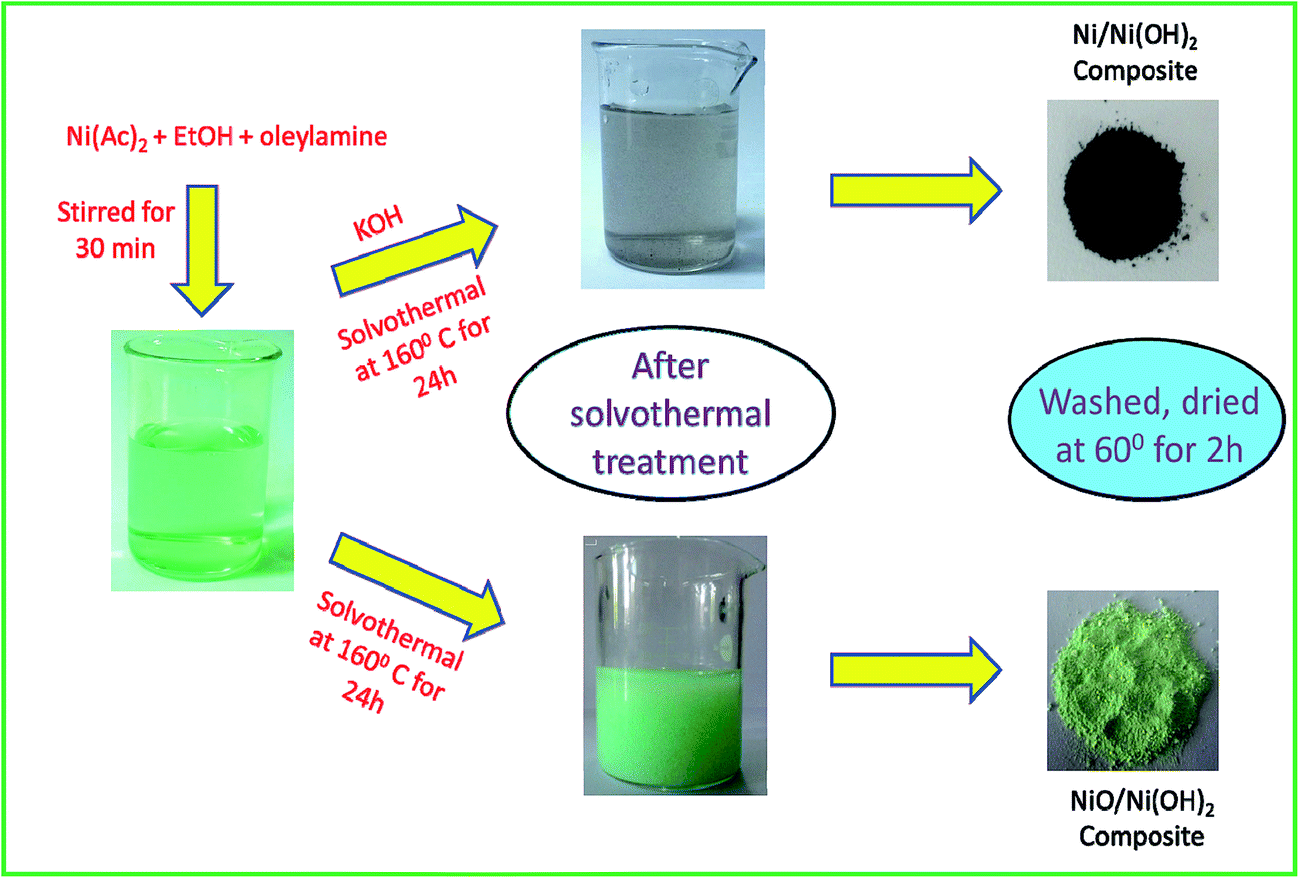
One step synthesis of Ni/Ni(OH) 2 nano sheets (NSs) and their application in asymmetric supercapacitors - RSC Advances (RSC Publishing) DOI:10.1039/C6RA26584G

Figure 5 from Thermodynamic model of Ni(II) solubility, hydrolysis and complex formation with ISA | Semantic Scholar

Nickel hydroxide precipitate formed by adding sodium hydroxide (NaOH) to a solution containing nickel ions. Nickel hydroxide (Ni(OH)2) is precipitated Stock Photo - Alamy
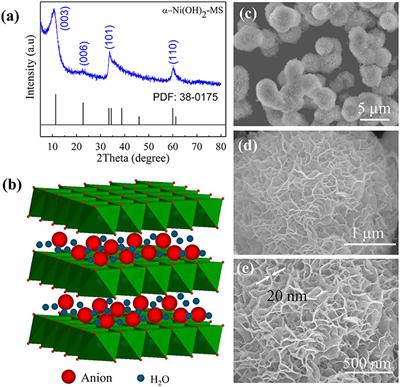
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

136. Calculate the molar solubility of Ni(OH)2 in 0.10M NaOH. The ionic product of Ni(OH)2 is 2.0x10-15 1) 2 x 10-13M 2) 10-12M 3) 2 x 10-'M 4) 4 x 10-13M following

NEET 2020 SOLUTION -Find out the solubility of Ni(OH)2 in 0.1 M NaOH . Given that the ionic product - YouTube

Strongly Coupled Ni/Ni(OH)2 Hybrid Nanocomposites as Highly Active Bifunctional Electrocatalysts for Overall Water Splitting | ACS Sustainable Chemistry & Engineering

Calculate the molar solubility of Ni(OH)2 in 0.10 M NaOH solution. The ionic product of Ni(OH)2 2.0 × 10^-15

Find out the solubility of Ni(OH)2 in 0.1 M NaOH.Given that the ionic product of Ni(OH)2 is 2×10-15 - YouTube
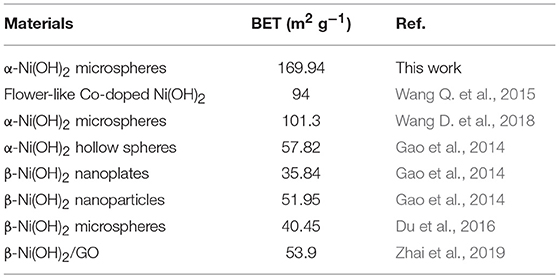
Frontiers | Facile Synthesis of Monodispersed α-Ni(OH)2 Microspheres Assembled by Ultrathin Nanosheets and Its Performance for Oxygen Evolution Reduction

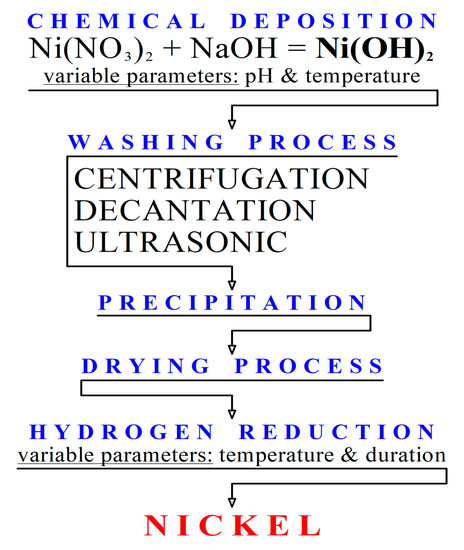

![Malayalam] Calculate the solubility of Ni(OH)2 in 0.1M NaOH solution. Malayalam] Calculate the solubility of Ni(OH)2 in 0.1M NaOH solution.](https://d10lpgp6xz60nq.cloudfront.net/ss/web-overlay-thumb/6531163.webp)